Magnesium is often overlooked in discussions of essential nutrients, many people assume it’s just a simple muscle relaxant, sleep-aid, perhaps a laxative, yet it is a powerhouse mineral that plays a critical role in numerous biological processes. Morley Robbins, a prominent advocate for nutritional health, emphasizes magnesium’s role in cellular function, energy production, and overall well-being. In this article, we will navigate the deep benefits of magnesium, with particular attention to magnesium bicarbonate, while drawing insights from various research findings and the teachings of many scientists + health advocates.
The Biological Significance of Magnesium:
1. Magnesium The Master Mineral:
Magnesium is the fourth most abundant mineral in the human body and is a crucial cofactor for over 3500+ (42%) of the body’s enzymatic reactions. It regulates various physiological functions, including:
– Energy Production: Magnesium is essential for ATP (adenosine triphosphate) synthesis, the molecule that stores and transfers energy within cells (Kirkland et al., 2018).
– Protein Synthesis: Magnesium Assists in ribosomal function, facilitating the translation of mRNA into proteins (Rude, 1998).
– DNA/RNA Synthesis: It stabilizes the structure of nucleic acids and is necessary for DNA replication and RNA transcription (Wang et al., 2016).
2. The Role of Magnesium in Enzyme Function
Magnesium acts as a cofactor for various enzymes involved in critical metabolic pathways. For instance, it plays a role in:
– Glycolysis and ATP production
– The Krebs Cycle
– The synthesis of neurotransmitters such as serotonin (Barbagallo & Dominguez, 2010)
3. Magnesium and Stress Response
Morley Robbins highlights magnesium’s role in mitigating the body’s stress response. Chronic stress depletes magnesium levels, potentially leading to a vicious cycle of anxiety and further depletion (Kirkland et al., 2018). Magnesium modulates cortisol production, which helps regulate stress and anxiety levels.
Let’s start with the Unique Benefits of Magnesium Bicarbonate
- Enhanced Bioavailability
Magnesium bicarbonate is a soluble form of magnesium that is more bioavailable than any other form, making it easier for the body to absorb and utilize (Friedman et al., 2017). The bicarbonate ion also plays a role in maintaining the body’s pH balance, which is essential for optimal cellular function.
- Alkalizing Properties
One of the remarkable features of magnesium bicarbonate is its alkalizing effect on the body. An overly acidic environment can lead to various health issues, including inflammation and metabolic disorders. Magnesium bicarbonate helps buffer acidity, promoting an optimal pH balance (Bach et al., 2016). This can be particularly beneficial for individuals with conditions like metabolic syndrome or chronic inflammation.
– Buffering System: Bicarbonate is a key component of the bicarbonate buffer system, which helps to stabilize the pH of blood (7.35-7.45) and other bodily fluids. This system involves the equilibrium between carbonic acid (H2CO3) and bicarbonate ions (HCO3-). When there is an excess of hydrogen ions (H+), bicarbonate can react with them to form carbonic acid, thus reducing acidity.
– Respiratory and Renal Function: The lungs and kidneys work together to maintain bicarbonate levels and, consequently pH balance. The lungs excrete carbon dioxide (Co2), which is in equilibrium with bicarbonate in the blood. The kidneys can excrete or reabsorb bicarbonate as needed, making this system highly adaptable.
- Cardiovascular Health
Research indicates that magnesium plays a critical role in cardiovascular health. Magnesium bicarbonate has been shown to support healthy blood pressure levels and heart function. A study by Zhang et al. (2016) indicated that adequate magnesium intake is associated with a reduced risk of hypertension. The bicarbonate component may also contribute to improved endothelial function, further promoting vascular health.
- Bone Health
Magnesium is integral to bone formation and maintenance. It aids in the regulation of calcium levels, promoting bone density and reducing the risk of osteoporosis (Rude, 1998). The bicarbonate form enhances magnesium’s absorption, potentially leading to improved bone health outcomes (Bach et al., 2016).
5. Muscle Function & Recovery
Athletes and active individuals often experience muscle cramps and fatigue due to magnesium deficiency. Magnesium bicarbonate can help alleviate these issues by ensuring adequate magnesium levels, promoting muscle contraction and relaxation (Kirkland et al., 2018.) Moreover its alkalizing effect can reduce lactic acid buildup during intense physical activity, enhancing recovery.
The Relationship Between Magnesium, Copper, and Iron
Robbins also emphasizes the importance of maintaining a delicate balance between magnesium, copper, and iron in the body. An imbalance can lead to various health issues. High levels of iron can inhibit magnesium absorption, while excess copper can lead to magnesium deficiency (Robbins, 2020). Therefore, ensuring adequate magnesium intake (in its most bioavailable form aka Mag Bicarbonate) is paramount for maintaining this balance and promoting overall health.
The Unique Benefits of Magnesium Bicarbonate: The Master Mineral Drink
Magnesium Bicarbonate is a chemical compound with the formula Mg(HCO3)2. It consists of magnesium ions (Mg2+) and bicarbonate ions (HCO3-). In its natural state, magnesium bicarbonate is typically found in water, especially in mineral springs, where it is formed from the dissolution of magnesium carbonate in the presence of carbon dioxide and water. This makes magnesium bicarbonate the most naturally occurring form of magnesium on the planet.
Magnesium Bicarbonate is the most bioavailable/absorbable form of magnesium on the planet, it is the only water soluble form of magnesium that can be naturally found in nature. It should be naturally found in our water supply, but thanks to acid rain, water treatment facilities, agricultural run off and various forms of environmental pollution, it has been virtually wiped out within the modern world, there are few exceptions such as European mineral water brands (Gerolsteiner). Bicarbonate is a powerful acid buffer in the human body and essentially acts as a transport for the magnesium, because it is water soluble, it simply passes through the stomach and absorbs directly through the small intestine – and actually gets where it needs to go, the cells.
It has been estimated that magnesium bicarbonate is roughly 80% or more bioavailable. Most other magnesium supplements are 4-30% bioavailable with very few being maybe 30-40% absorbable. This means you get more bang for your bucks, and magnesium bicarbonate allows you to uptake more magnesium before experiencing side effects.
Alkaline Water vs. Alkaline Bicarbonate Water
Alkaline water —including alkaline ionized water— is very different from water that has an alkaline pH due to the presence of bicarbonates. Alkaline ions affect the pH of water but they cannot neutralize acids. (Read the article on alkaline water.) Most alkaline water products have few bicarbonates, little buffering capacity and almost no ability to neutralize acidic waste. On the other hand, bicarbonate water is mildly alkaline yet it has the ability to neutralize acids.
Bicarbonates in the Stomach
In the stomach, bicarbonate ions pass almost immediately into the bloodstream in exchange for chloride ions to make hydrochloric acid (HCl). Although it sounds counter-intuitive, drinking bicarbonate water on an empty stomach actually stimulates the production of stomach acid (HCl). It is an ideal drink first thing in the morning and before meals—as long as adequate time passes before the consumption of food. Bicarbonate water actually prepares the stomach for digestion and helps to overcome the waning production of HCl that plagues older individuals. While such knowledge has nearly disappeared from medical texts, the Materia Medica (1918 edition) had this to say about the effects of sodium bicarbonate in the stomach:
The effect of an alkali in the stomach will vary according to the nature of the stomach contents at the time of administration. In the resting period (after food is digested) sodium bicarbonate dissolves mucus and is absorbed as bicarbonate into the blood, to increase its alkalinity directly. In the digestive period it reduces the secretion of gastric juice, neutralizes a portion of the hydrochloric acid, liberates the carminative carbon dioxide gas, and is absorbed as sodium chloride. The time of administration must, therefore, be chosen with a definite purpose. In continuous hyperacidity and in fermentative conditions a dose an hour before meals will tend to prepare the stomach for the next meal.
The use of bicarbonates is not new. What is new is the use of magnesium bicarbonate in water to enhance bicarbonate reserves, to stabilize the structure of the water, AND to supply much needed magnesium in a biologically available form.
Decomposition:
When magnesium Bicarbonate is subjected to heat or when the pressure of carbon dioxide is reduced (for example, when water is boiled), it decomposes into magnesium carbonate, carbon dioxide and, and water.
Magnesium bicarbonate is made by vigorously mixing Magnesium hydroxide cold, clean water, and a heaping injection of Co2 then letting it rest, and shaking it some more. What a lot of companies and “specialists” don’t mention is that the chemistry reports literally showcase how stubborn the process is. It took us 1000’s of attempts to finally find a process that completely turns a high dosage clear while still maintaining high amounts of carbonation (which ensures that it does not turn back into magnesium carbonate). Our process is unique like no other, the issue is that it has been challenging for us to upscale and thus we find ourselves out of stock more often than we would like. Because of this process, we believe ours is the most absorbable, electrifying and hydrating form of magnesium bicarbonate on the market.
One quick thing I want to mention before diving back into more of the science is the fact that the quality of your magnesium hydroxide matters a lot! Our hydroxide is sourced from the dead sea and we believe there is no other comparison on the planet, but unfortunately we are unable to resell our hydroxide. Hopefully in the future we will find an equally high quality source of magnesium hydroxide. This is essential one, because if you are going to make it yourself it has to be very pure and fine, most people do not realize that the majority of magnesium hydroxide sources on the market are used for water treatment processes to adjust pH levels and to provide some magnesium, which is beneficial for plant growth and can improve the taste of drinking water. Trying to use these low grade forms of hydroxide is very challenging to fully convert into a finished product (magnesium bicarbonate). If you have any cloudiness, this indicates that the magnesium hydroxide was not fully converted and could potentially yield some nasty side effects (cloudiness is not crystallization this is different).
So what also makes our magnesium bicarbonate better than making it yourself at home is not only the fact that we use high quality commercial grade equipment and hours upon hours worth of vigorous shaking to ensure full conversion, but also price. Yes price, some people think our magnesium bicarbonate is expensive and a cheaper alternative would be to make it at home with a subpar form of hydroxide. We will not name brands, because we like to stay classy, but some high end forms of magnesium hydroxide are so expensive that if you were to use the same amount of hydroxide that we use (using the competitors source of hydroxide) and you try making your own magnesium bicarbonate with the comparable process and added minerals that we use (sodium and potassium bicarbonate), it ends up costing the same amount. Plus you receive a beautiful swing top glass bottle we ensure the product is fully converted and you waste no time. Add in discounts and we end up being more cost effective.
No, your soda stream or plastic Perrier water bottle does not compare to our state of the art process. Never will, trust us, we tried LOL. Yes this is laugh out loud worthy, because many are misled by this bogus information. We do want to teach our methods very soon once we can sell our own hydroxide source, but again we have signed agreements, unfortunately. It is what it is, I digress. One more thing I want to reiterate, Magnesium bicarbonate is very unstable and easily decomposes and converts into magnesium carbonate, this is why the in-home process does not yield the highest quality form of magnesium, not even remotely close.
Also, other forms of magnesium bicarbonate brands sometimes may appear to have a higher dose, this is misleading because a lot of these products from our competitors are cloudy, this is UNCONVERTED magnesium hydroxide. We like to call this diarrhea juice! If you like flushing out your minerals and sitting on the toilet multiple times a day, be our guest. There’s levels to this process and it takes time to figure it out, we’ve put in the time and we are constantly evolving and refining our process as well as updating the formula to make it the best of the best supplements to ever be placed on the market.
So we discussed that the quality of your magnesium hydroxide source matters, the process matters a lot (which is challenging to replicate in-home), and simply placing it in a water bottle does not ensure the highest quality yield or stabilization for the matter. You have to have special quality equipment to ensure enough Co2 is stabilizing the end product, NOT JUST CONVERTING. This is key. This is what separates us from the pack.
Now, let’s discuss what makes Magnesium Bicarbonate the best form of magnesium on the planet. That is the very ion it is attached to, the bicarbonate ion. Bicarbonate salts, particularly those like sodium bicarbonate (baking soda) and magnesium bicarbonate, are increasingly recognized for their potential health benefits, particularly in terms of pH regulation, metabolic support, stimulation of the hypothalamus for short term and long term memory, pancreatic function (your pancreas stores bicarbonate salts) kidney + liver function and more while limiting digestive distress. I know we already discussed magnesium bicarbonate benefits a little bit earlier, but here is an even deeper exploration into the science underlying bicarbonate salts and their healing properties..
1. Thyroid and Bicarbonate:
– Dr. Ray Peat PhD emphasizes the connection between proper thyroid function and metabolic health. He notes that bicarbonate can support thyroid function by ensuring that the metabolic pathways requiring thyroid hormones operate efficiently. Magnesium, which often accompanies bicarbonate in certain mineral waters (Gerolsteiner), is also crucial for thyroid hormone activation.
2. Carbon Dioxide and Bicarbonate Equilibrium:
– The bicarbonate buffering system is closely linked to the respiratory system. When CO2 levels increase (e.g., during exercise), bicarbonate can help mitigate the resultant acidity. This equilibrium is vital for maintaining oxygen transport and utilization in tissues.
3. Chemical Composition and Structure:
– Bicarbonate salts are composed of bicarbonate ions (HCO3-) combined with various cations (positively charged ions), such as sodium (Na+) in sodium bicarbonate or magnesium (Mg2+) in magnesium bicarbonate. The bicarbonate ion itself is a weak acid and a vital component of the body’s buffer systems, which help maintain stable pH levels in various biological fluids.
4. Metabolic Support
– Energy Production: Bicarbonate ions are involved in the Krebs cycle (citric acid cycle), a crucial metabolic pathway for energy production in cells. This cycle relies on a delicate balance of acids and bases, and bicarbonate helps maintain that balance. Without adequate bicarbonate (or magnesium), energy production can be compromised.
– Detoxification: By neutralizing excess acids in the body, bicarbonate can aid detoxification processes, potentially reducing the burden on the liver and kidneys. This can help the body maintain optimal function and health.
5. Stress and Mineral Imbalance:
– Morley Robbins’ framework often includes the direct impact of stress (physical, chemical, & emotional sources) on mineral imbalance. Stress can deplete magnesium levels and disrupt bicarbonate homeostasis. By restoring bicarbonate through dietary means (such as through mineral water rich in bicarbonate or a high quality magnesium bicarbonate formula like ours), individuals may support their magnesium status and overall mineral balance, addressing some of the negative effects of chronic stress. This means the Bicarbonate ion itself supports magnesium absorption and our overall magnesium levels! No other form does that.
6. Gut Health & pH Balance
Bicarbonate plays a crucial role in maintaining acid-base balance in the body. The potential healing properties of bicarbonate salts are supported by various studies and anecdotal evidence:
– Acid Base Disorders: Bicarbonate therapy is often used in medical settings to treat metabolic acidosis, a condition where the body produces excess acid or the kidneys are not removing enough acid from the body. Many people may experience varying levels of this unknowingly.
– Gastrointestinal Health: Sodium & Magnesium Bicarbonate is commonly used to relieve heartburn and indigestion by neutralizing stomach acid, providing symptomatic relief. This is why we recommend consuming magnesium bicarbonate AWAY from food, at least 45 minutes before and preferably 2 hours after. Even though it will neutralize stomach acid, some research has shown that it will actually stimulate HCL production over time! Magnesium does indeed help support digestive health by promoting regular bowel movements and preventing constipation. Magnesium bicarbonate can enhance gut health, which in turn supports the body’s natural detoxification process.
– Exercise and performance: Some research suggests that bicarbonate supplementation can enhance athletic performance by buffering lactic acid buildup during high-intensity exercise, which may reduce fatigue and improve endurance.
– Systemic Alkalinity: Magnesium bicarbonate is associated with promoting systemic alkalinity, which may have various health benefits, including improved cardiovascular health, better bone density, and enhanced cellular function.
7. Hydration and Electrolyte Balance:
– Bicarbonate ions help maintain hydration and electrolyte balance. Adequate hydration is critical for cellular functions, metabolic processes, and overall health. Magnesium bicarbonate, in particular, can contribute to deep cellular hydration and greatly support electrolyte balance unlike any other form on the market, it’s the form the body most easily recognizes and utilizes.
8. Impacts on Inflammation and Disease:
– Anti Inflammatory Effects: Some studies suggest that bicarbonate may have anti-inflammatory properties, potentially benefiting conditions characterized by chronic inflammation, such as arthritis.
– Cancer Research: Preliminary research indicates a possible role for bicarbonate in cancer therapy by creating an alkaline environment that may inhibit tumor growth. However, this area is still under investigation and should be approached with “caution”. As we know with all “cancer research”. 😉
9. Natural Sources and Availability
Bicarbonate salts can be found in various natural sources, including:
– Mineral Waters: Natural mineral waters often contain bicarbonate, providing a source of this compound in a bioavailable form, we recommend drinking Gerolsteiner.
– Diet: While bicarbonate itself is not typically consumed directly, foods that promote alkalinity can help the body maintain bicarbonate levels.
The Reason WHY Supplementing with Magnesium Bicarbonate Should be a Priority for Everyone:
In today’s world, where pollution and nutrient deficiencies are prevalent, the need for magnesium bicarbonate is more pressing than ever. This Master Mineral plays a vital role in various bodily functions and offers unique benefits as we have discussed. One more very important reason why magnesium needs to be a priority in everyone’s supplementation regimen, is simply for its powerful detoxification and protection against heavy metals.
In a polluted/deficient world, magnesium has become more important than ever before.
1. Nutrient Deficiency
Many people today have diets that are deficient in essential nutrients, including magnesium. Factors such as soil depletion, processed foods, and lifestyle stressors contribute to this deficiency. According to the National Institutes of Health, a significant portion of the population does not meet the recommended daily intake of Magnesium (NIH, 2021). This deficiency can lead to numerous health issues, including muscle cramps, fatigue, anxiety, stress, depression, and weakened immunity.
2. Pollution and Heavy Metals
Our environment is rife with pollutants and heavy metals, such as lead, mercury, cadmium, and arsenic. These substances can accumulate in the body and cause a range of health problems, from neurological damage to cardiovascular disease. The body has natural detoxification mechanisms, but these can be overwhelmed by the sheer volume of toxins present in our environment.
How Magnesium Bicarbonate Supports Detoxification and Protection…
Chelation of Heavy Metals
Magnesium has chelating properties, meaning it can bind to heavy metals and facilitate their excretion from the body. Chelation therapy is a medical treatment used to remove heavy metals, and magnesium bicarbonate can serve as a natural alternative or adjunct to this therapy. By binding with metals like lead and mercury, magnesium bicarbonate helps to detoxify the body, reducing the toxic burden on the organs (Schoenfield et al., 2013)
Conclusion:
In a world increasingly burdened by pollution and nutrient deficiencies, magnesium bicarbonate emerges as a vital player in promoting health and detoxification. Its unique properties, including the ability to electrify the body (electrolyte), facilitate mineral and nutrient absorption while enhancing metabolic function, enzyme production, chelate heavy metals, support deep cellular hydration, energy production, reduce systemic inflammation, acid buffering effects, organ/detoxification support for the gut, kidneys, liver and pancreas.The scientific mechanisms underpinning the benefits of bicarbonate, especially in the context of magnesium, suggest that these compounds may indeed be among the most healing and health-promoting nutrients available.
References:
– Bach, J. F., et al. (2016). “The role of bicarbonate in the acid-base balance of the body.” American Journal of Clinical Nutrition.
– Barbagallo, M., & Dominguez, L. J. (2010).
“Magnesium and aging.” Current Pharmaceutical design, 16(7), 832-839
– Friedman, S. M., et al. (2017). “Bioavailability of magnesium from different sources.” Nutrients, 9(5), 477.
– Kirkland, A. E., et al. (2018). “Magnesium in Health and Disease.” Nutrients, 10(2), 186.
– Rude, R. K. (1998). “Magnesium deficiency: a cause of heterogeneous disease in humans.”
Journal of the American College of Nutrition, 17(5), 441-446.
– Robbins, M. (2020). “Magnesium: The Forgotten Nutrient.” Nutritional Perspectives.
– Wang, Y., et al. (2016). “Magnesium: A key player in DNA repair.” Free Radical Biology and Medicine, 100, 41-48.
– Zhang, X., et al. (2016). “Dietary Magnesium intake and risk of hypertension: a systematic review and meta-analysis.” BMC Public Health, 16, 1201.
By understanding the profound impact of magnesium and its bicarbonate form, individuals can take proactive steps toward better health and well-being. As always, consulting with a healthcare professional is advisable before making significant changes to dietary or supplementation practices. None of this intended to be used as medical advice.
USE CODE MAG20 FOR 20% OFF YOUR FIRST ORDER AT MITIGATESTRESS.COM FOR THE WORLD’S BEST MAGNESIUM BICARBONATE!

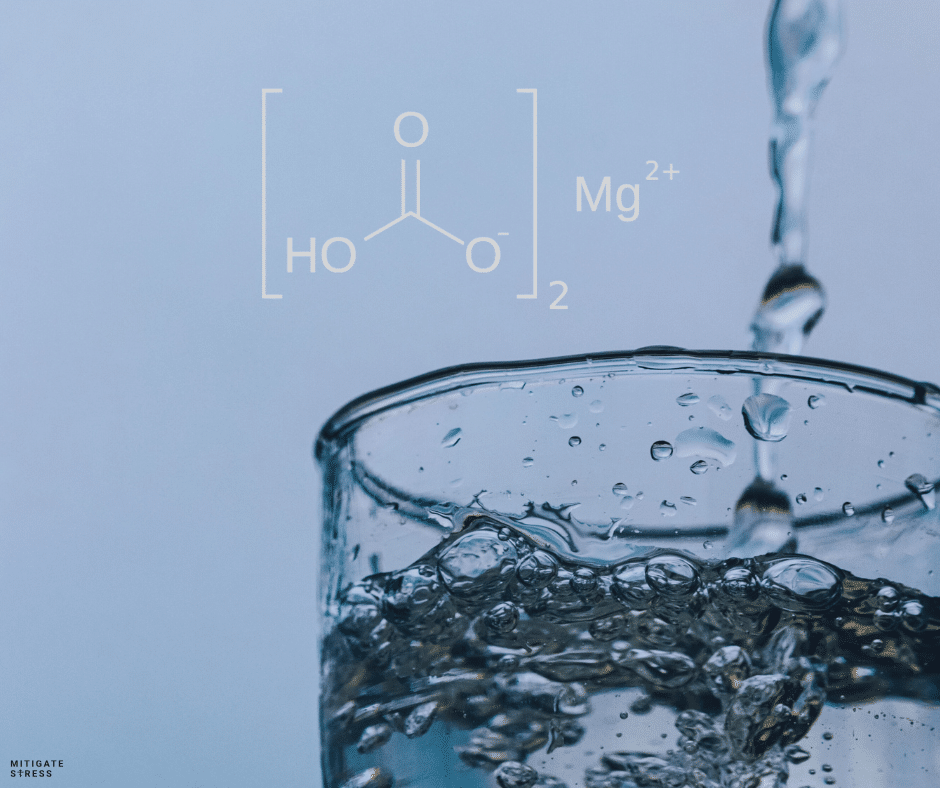




0 Comments