Abstract
Heavy metal toxicity remains a significant public health concern, with exposure linked to various health issues, including neurological disorders, cardiovascular diseases, and compromised immune function. Traditional detoxification methods often focus on chelation therapy, which can have adverse effects. This review explores the potential role of magnesium bicarbonate as a therapeutic agent in heavy metal detoxification, highlighting its biochemical properties, mechanisms of action, and supporting literature, including insights from researchers like Ray Peat, PhD. and Morley Robbins.
Here at Mitigate Stress, we still believe that the accelerated evolution of modern diseases is a result of toxicity overload, not just germs. This toxicity is primarily not just chemicals but heavy metals such as lead, mercury, arsenic, and cadmium, which accumulate in the body, leading to acute and chronic health conditions. Detoxification strategies often employ chelating agents; however, these approaches can have significant adverse effects and may not effectively eliminate all toxic metals. Magnesium bicarbonate, a soluble form of magnesium, has garnered attention for its potential benefits in supporting detoxification processes due to its unique properties. This article delves into the scientific basis behind magnesium bicarbonate’s role in detoxifying heavy metals, examining physiological mechanisms, and integrating perspectives from notable researchers.
Magnesium: A Vital Mineral
Magnesium (Mg) is the fourth most abundant mineral in the human body and plays a crucial role in over 3500 enzymatic reactions (42% of the body’s total enzymatic activity, according to Morley Robbins). It is pivotal in energy production, protein synthesis, and DNA/RNA synthesis. Moreover, magnesium is essential for the synthesis of glutathione, a key antioxidant involved in detoxification pathways (Serefko et al., 2016).
According to Morley Robbins, an advocate for magnesium’s importance, cellular magnesium influences various metabolic pathways, including those responsible for managing oxidative stress and heavy metal elimination (Robbins, 2020).
Magnesium Bicarbonate: Properties and Mechanisms
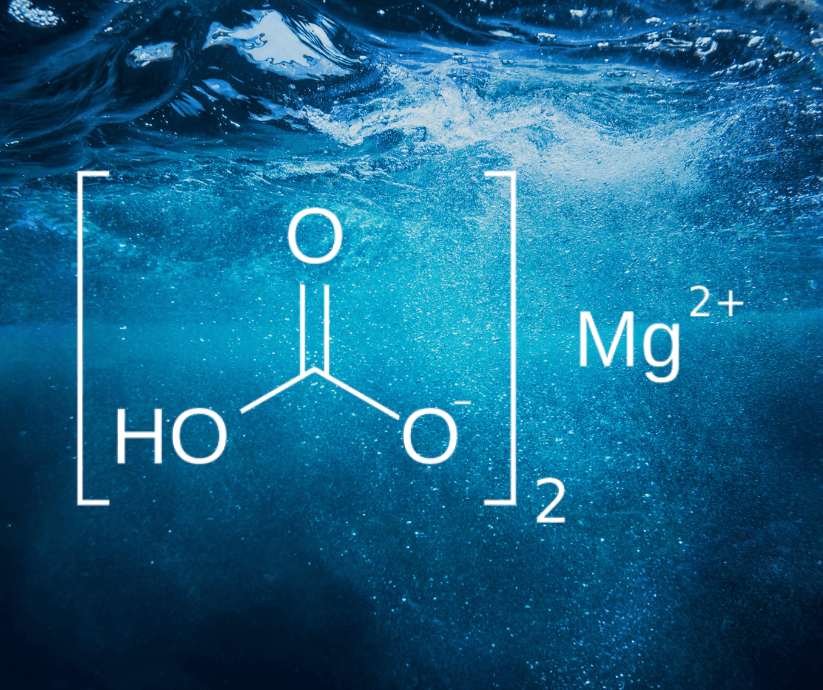
Magnesium Bicarbonate (Mg(HCO3)2) is a water-soluble salt that dissociates in aqueous solutions to release magnesium and bicarbonate ions. The unique attributes of magnesium bicarbonate include:
- Bioavailability: Magnesium bicarbonate is highly bioavailable, making magnesium readily available for physiological functions, including detoxification (Cleveland Clinic, 2021). Most forms of magnesium are 4-30% absorbable. The rest simply irritates the intestines, causing the body to purge it, which pulls more water and electrolytes, inevitably leaving the body dehydrated and depleted. Our magnesium bicarbonate is roughly 75-85% absorbable, making it the most bioavailable form of magnesium on the planet. This means you uptake more magnesium before experiencing nasty side effects. This also means you save money!
- Alkalinity: The bicarbonate ion contributes to maintaining an optimal pH balance in the body. Most people assume alkaline water is what makes it “alkalizing” when in reality bicarbonates are what truly create an alkaline environment, which may enhance the body’s ability to excrete heavy metals through urine (Bicarbonate Therapy Study, 2019).
- Antioxidant Properties: Magnesium plays a pivotal role in the synthesis of glutathione, while bicarbonate can mitigate oxidative stress, collectively supporting detoxification processes (Khan et al., 2017).
Mechanisms of Heavy Metal Detoxification
Chelation and Excretion: Magnesium can compete with heavy metals for binding sites in biological systems, potentially reducing metal absorption and facilitating their excretion (Sharma et al., 2020). The bicarbonate component may aid in the solubilization and elimination of heavy metals through urine. Magnesium has chelating properties, meaning it can bind to heavy metals and facilitate their excretion from the body. Chelation therapy is a medical treatment used to remove heavy metals, and magnesium bicarbonate can serve as a natural alternative or adjunct to this therapy. By binding with metals like lead and mercury, magnesium bicarbonate helps to detoxify the body, reducing the toxic burden on the organs (Schoenfield et al., 2013)
The issue with chelation therapy is that it can release these heavy metals back into the bloodstream causing them to recirculate and wreak havoc on our physiology before even getting expelled from the body safely. This is why Dr. Aajonus Vonderplanitz, PhD, is so adamant about using raw saturated fats as a binding agent to prevent the recirculation of these extremely caustic compounds.
Glutathione Production: Magnesium is a cofactor for the enzyme gamma-glutamylcysteine synthetase, which catalyzes the first step in glutathione synthesis (Serefko et al., 2016). Enhanced glutathione levels improve the body’s capacity to neutralize reactive oxygen species and facilitate the conjugation and excretion of heavy metals.
Kidney Function Support: Adequate magnesium levels are essential for optimal kidney function, which is crucial for filtering toxins and heavy metals from the bloodstream. Magnesium bicarbonate may support renal health and enhance detoxification efficiency (Baker et al., 2019).
Perspectives from Ray Peat
Ray Peat’s work emphasizes the importance of metabolic health and the role of magnesium in reducing stress on the body. He posits that magnesium can mitigate the effects of heavy metals by enhancing energy production and reducing oxidative stress (Peat, 2016). Peat’s focus on the metabolic processes influenced by magnesium suggests that ensuring adequate magnesium levels may bolster the body’s innate detoxification mechanisms.
Clinical Implications and Further Research
While preliminary evidence supports the role of magnesium bicarbonate in heavy metal detoxification, further clinical studies are necessary to establish definitive protocols and therapeutic dosages. Investigating the interplay between magnesium, bicarbonate, and heavy metal detoxification may offer insights into novel treatment strategies for individuals with heavy metal exposure.
Conclusion
Magnesium bicarbonate presents a promising avenue for supporting heavy metal detoxification due to its bioavailability, alkalizing properties, and ability to enhance antioxidant defenses. Although more research is always needed, integrating magnesium bicarbonate into detoxification regimens may provide a safer and more effective alternative to traditional chelation therapies. Future studies should focus on elucidating the mechanisms by which magnesium bicarbonate influences heavy metal excretion and overall metabolic health. You can use code MAG20 for 20% off your next order by clicking here.
References
- Baker, H. D., et al. (2019). “Magnesium and kidney function: A review.” Journal of Renal Nutrition, 29(5), 319-327.
- Bicarbonate Therapy Study (2019). “Bicarbonate Therapy: A Systematic Review.” Clinical Biochemistry, 63, 1-10.
- Cleveland Clinic. (2021). “Magnesium: Health Benefits, Sources and Deficiency.”
- Khan, M. I., et al. (2017). “Antioxidant properties of magnesium: A review.” Journal of Pharmacy and Bioallied Sciences, 9(1), 1-5.
- Peat, R. (2016). “Nutrition for metabolism: The importance of magnesium.” Ray Peat’s Website
- Robbins, M. (2020). “Magnesium: The missing link in detoxification.” The Magnesium Advocacy Group.
- Serefko, A., et al. (2016). “Magnesium and glutathione: A review.” Journal of Physiology and Pharmacology, 67(4), 485-491.
- Sharma, S., et al. (2020). “Role of magnesium in heavy metal detoxification: A review.” Environmental Toxicology and Pharmacology, 80, 103386.
Note:
This article serves as a synthesis of various studies and expert opinions. For specific health concerns or before starting any supplementation, individuals should consult with a healthcare professional.
Also, feel free to use code MAG20 at MITIGATESTRESS.com for a mega discount on the world’s best magnesium. You can also schedule a 30-minute consultation with us. God bless!




0 Comments